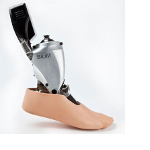
Cogmedix today announced that it is a named supplier to the BiOM Ankle Prosthetic, a device chosen as a finalist in the rehabilitation and assistive-technology product category of the 2012 Medical Design Excellence Awards (MDEA) competition, submitted by iWalk Inc. ( Bedford, MA ). Cogmedix is an ISO 13485 certified and FDA compliant medical device con tract manufacturer and has been the contract manufacturer for iWalk’s BiOM Ankle since its production release.
Cogmedix is a wholly owned subsidiary of Coghlin Companies, a fourth generation privately owned contract engineering and manufacturing company located in central Massachusetts.
Medical Design and Diagnostic Industry (MD+DI), the global MedTech authority providing the medical device industry with the latest news, information, and in-depth analysis, announced the finalists, and their suppliers, in the 2012 MDEA competition in its April issue published this week.
Sponsored by MD+DI and organized by UBM Canon, the MDEA competition is the premier awards program for the medical technology community. It recognizes the achievements of medical device manufacturers, their suppliers, and the many people behind the scenes — engineers, scientists, designers, and clinicians — who are responsible for the grou ndbreaking innovations that are changing the face of healthcare. MDEA – finalist and winning entries excel in the areas of product innovation, design and engineering achievement, end-user benefit, and cost-effectiveness in manufacturing and healthcare delivery.
A comprehensive review of the entries was performed by an impartial, multidisciplinary panel of third-party jurors with expertise in biomedical engineering, clinical practice, diagnostics, human factors, industrial design, manufacturing, and medicine. Entries are evaluated on the basis of their design and engineering features, including innovative use of materials, user-related functions that improve healthcare delivery and change traditional medical attitudes or practices, features that provide enhanced benefits to the patient, and the ability of the product development team to overcome design and engineering challenges so that the product meets its clinical objectives. The 2012 MDEA Jury selected 41 finalists in 10 medical product categories.
“It’s an honor to contribute to the success of such a wonderful product. We are proud that our contributions to the BiOM Ankle have been recognized,” said Matt Giza, Vice President and General Manager of Cogmedix. “We are confident that our continued attention to compliance and quality will help iWalk continue changing lives.”
Winning products will be announced at the MDEA Presentation Ceremony held on Wednesday, May 23, 20, 2012 at 4:30 pm during a cocktail reception inside the Philadelphia Marriott Downtown hotel. The ceremony is held in conjunction with the MD&M East Event ( www.MDMEast.com ) at the adjacent Pennsylvania Convention Center, May 22 – 24, 2012.
During the MDEA Presentation Ceremony on May 23, 2012:
- The 2012 MDEA finalists and their suppliers will be honored for their innovative contributions to the medical device industry.
- Up to one Bronze, one Silver, and one Gold – winning product will be announced in each category.
- One Gold-winning product will be awarded “Best-in-Show”.
- The NCIIA will announce the winners of the 2012 BMEidea, a biomedical engineering competition for university students.
- Famed inventor Dr. Thomas Fogarty will be presented with the prestigious MDEA Lifetime Achievement Award.