Paul Dunleavy, Senior Vice President of Engineering
Medical device product development and commercialization can be challenging, especially when technology innovation is critical to developing your product. Regulatory compliance with a rigorous Design Control process further constrains commercialization. The success of a program strongly depends on financial backing, corporate sponsorship, team competency and dedication, and project structure and process. Market need and acceptance is assumed to be present.
Since we are in a regulated medical device industry, we are required to have a Waterfall product development process. The Design Controls process contains major stages that include user needs, design input, design process, design output, verification, and validation which naturally fall within the Waterfall process. This process is a more rigorous approach with linear and well-structured stage gates. The Waterfall process makes it easier to identify the full scope of work and develop a structured project plan. The difficulty with this route is that it is less flexible than an Agile product development process and is difficult to plan for innovation.
Agile product development is an alternative process but is more difficult to maintain compliance with Design Controls and manage scope creep. As Agile emphasizes individual interactions, technology, and fast changes, Waterfall emphasizes formal process, scope management, and documentation. While Agile was developed for software development, it is also more suited to technology innovation.
Cogmedix’s Design Control process has four distinct phases of development (see flow diagram). As a formal stage gate process, all deliverables are reviewed and accepted before entering the next phase.
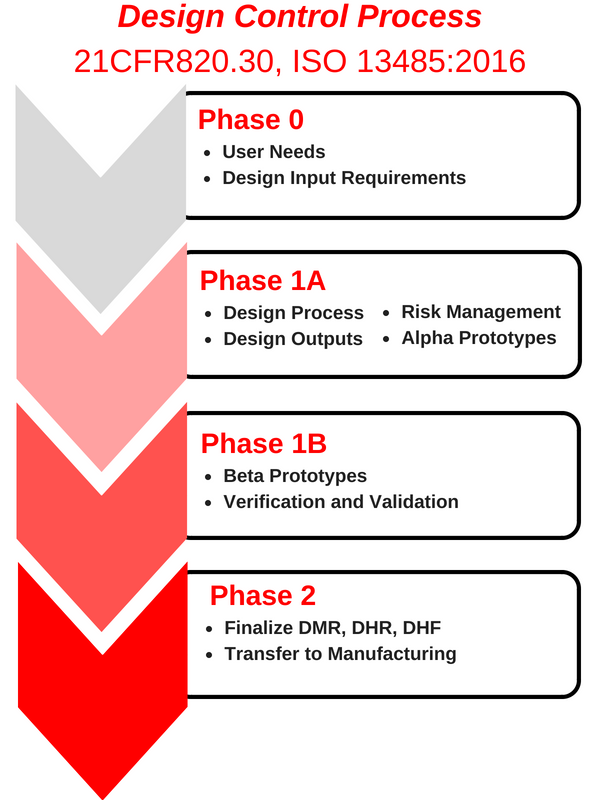
A method to maintain compliance while fast-tracking technology innovation is to structure the overall project under Design Control while concurrently innovating core technology. As an example, the system architecture and system development can be developed under the formal Design Control process. Core technology unique to the industry can go through rapid innovation and the design iteration process concurrently. You should ensure that the core technology is designed in a modular manner with well-defined mechanical and electrical interfaces. Once the core technology has matured sufficiently, it can be integrated into the system and commercialization can proceed through the rest of the Design Control process. Requirements may need to be revised once the innovation is completed.
Good project leadership and team structure are essential to the success of the program. Cogmedix utilizes a matrix organization with program managers leading the project and dedicated engineers developing the product and manufacturing process. Matrix organization allows separation of roles and responsibilities, allowing deeper focus in core disciplines resulting in better overall deliverables and program management.
Clear and agreed upon requirements are also essential to the success of the program. Some requirements to consider and document formally are:
- Clinical performance requirements
- Usability requirements
- Market and economic requirements
- Product safety requirements
- Product system performance requirements
Cogmedix has the expertise and capabilities to assist our customers at any phase of the commercialization process, and is dedicated to providing an exceptional customer experience.


 Contact Cogmedix today to discuss your organization's needs and aspirations. Cogmedix can help you grow, thrive and profit.
Contact Cogmedix today to discuss your organization's needs and aspirations. Cogmedix can help you grow, thrive and profit.